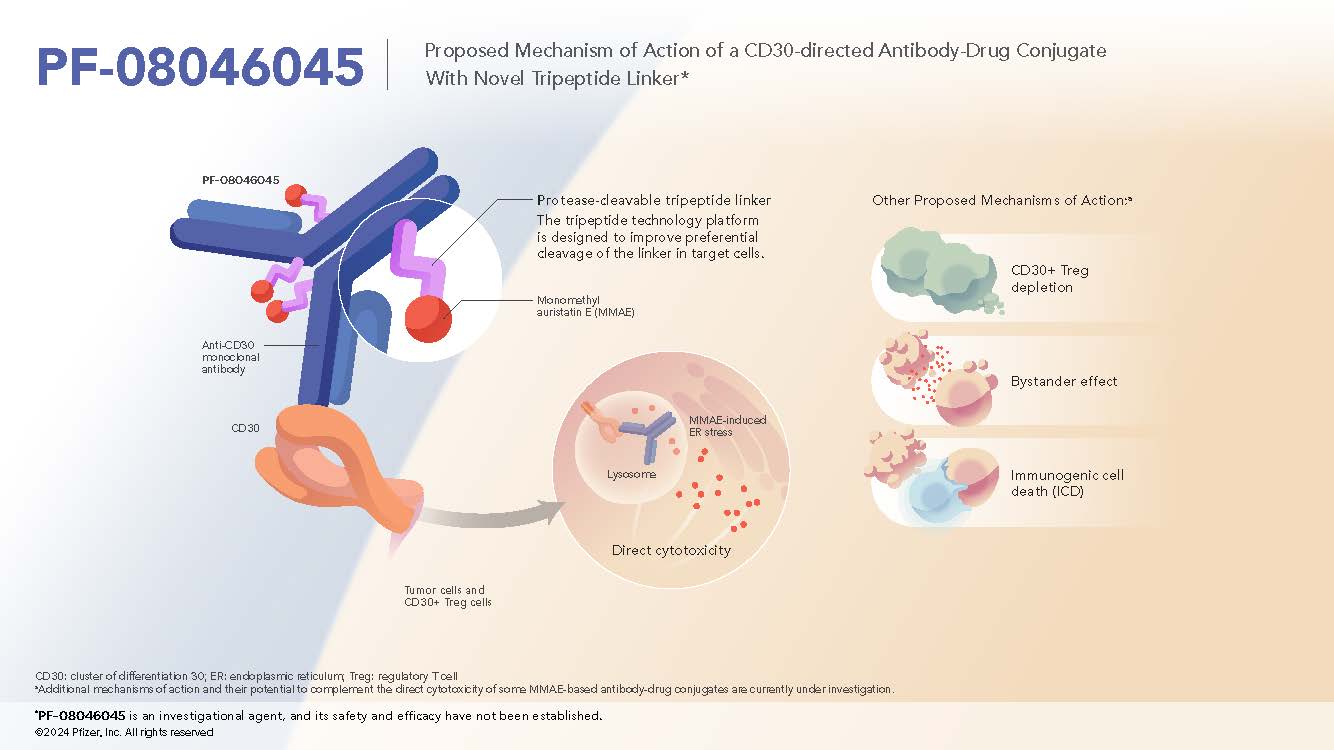
CD30-directed Antibody-Tripeptide MMAE Conjugate
PF-08046045 is an investigational compound. Its safety and efficacy have not been established
Overview + Rationale
- PF-08046045 is a CD30-directed ADC and utilizes the novel cleavable tripeptide linker.1
- CD30 is expressed in many B- and T-cell lymphomas while having limited expression in normal tissue2
Mechanism of Action
Stage of Development
Lymphomas
Phase 1 Monotherapy

 Back
Back
