Tegtociclib (PF-07104091)
Tegtociclib | PF-07104091 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
- The cyclin-dependent kinases (CDKs) are a family of enzymes that play a major role in controlling entry into the cell-cycle
- Treatments using CDK inhibition have focused on targeting cyclin-dependent kinases 4 and 6 (CDK4/6); however, primary and secondary resistance to CDK4/6 inhibition represent a challenge in the treatment of HR+ breast cancer
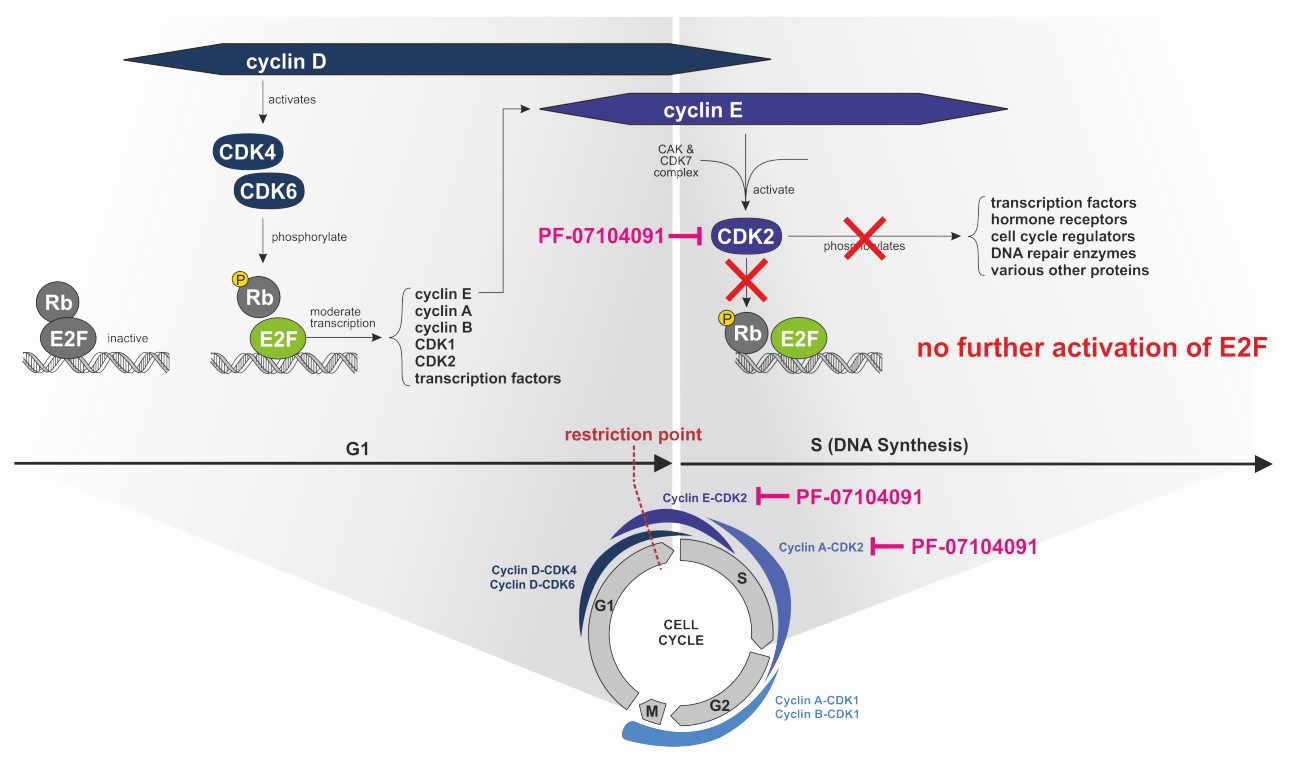
- CDK2 is another regulator of cell cycle progression that is activated by cyclins E and A, and also phosphorylates retinoblastoma (Rb)
- Besides Rb, CDK2 was shown to phosphorylate of a wide variety of target proteins, some of which are known to play a role in cancer pathogenesis
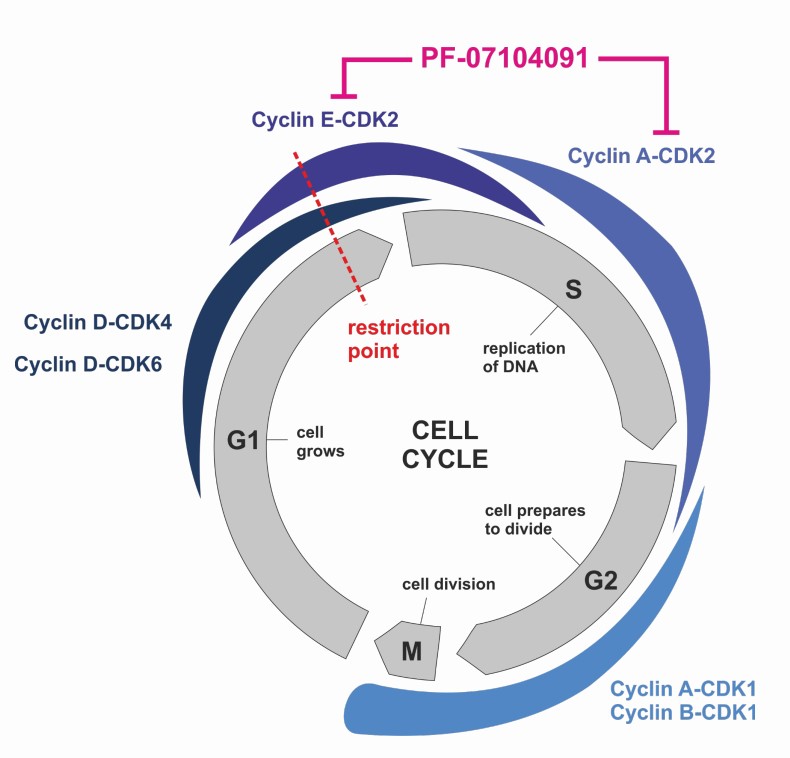
- CDK2 activity is largely dispensable for normal development in mice, but it is associated with tumor growth in multiple cancer types and emerging evidence suggests that selective CDK2 inhibition may provide a therapeutic effect against certain tumor types
- Tegtociclib | PF-07104091 is a CDK2-selective inhibitor under clinical investigation with potential antineoplastic activity
Mechanism of Action
Tegtociclib | PF-07104091 selectively binds to, and inhibits the activity of CDK2 which may lead to cell cycle arrest through reduced phosphorylation of Rb, and other phosphotargets
Inhibition
Stage of Development
HR+/HER2- Metastatic Breast Cancer
Phase 1/2A Monotherapy and Combination*
Phase 1B/2 Combination*
Phase 1B/2 Combination*

 Back
Back
