Disitamab vedotin (PF-08046051)
Overview + Rationale
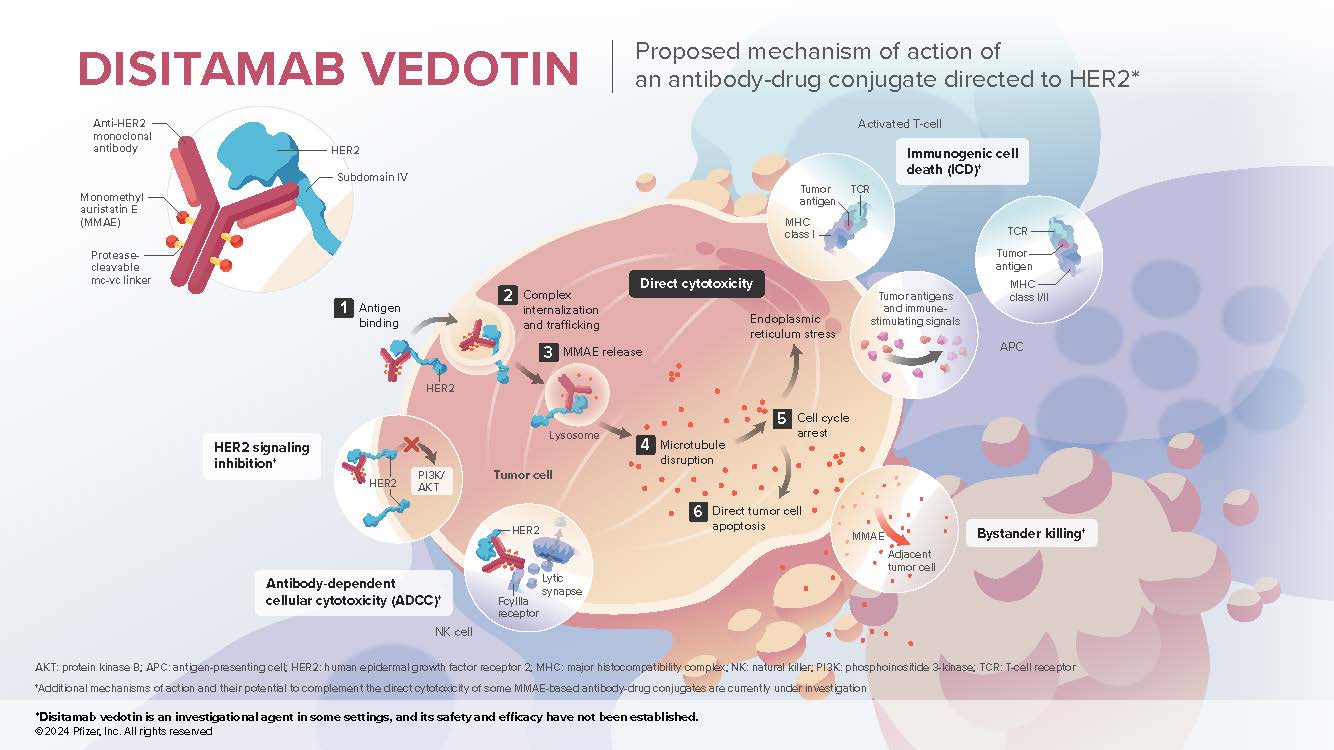
- Disitamab vedotin (DV) is an investigational antibody-drug conjugate that contains 3 components: a recombinant, humanized, high affinity IgG1 monoclonal antibody directed to human epidermal growth factor receptor (HER2), a microtubule-disrupting agent MMAE (monomethyl auristatin E), and a protease-cleavable mc-vc (maleimidocaproyl-valine-citrulline) linker that covalently attaches MMAE to the antibody, which enables preferential release of MMAE within target cells
- DV binds to a distinct epitope on subdomain IV of the HER2 extracellular domain1,4
- HER2 is a receptor tyrosine kinase2 that is overexpressed or amplified in multiple cancers, including breast, bladder, gastroesophageal, colorectal, ovarian, and lung3
- DV is optimized for enhanced internalization and delivery of cytotoxic MMAE to target cells
Partner: RemeGen Co. Ltd.
Mechanism of Action
Stage of Development
Disitimab vedotin is being investigated in the tumor types shown below. Safety and efficacy for the uses listed below have not been established.
Urothelial Cancer with HER2 Expression
Phase 2 Monotherapy and Combination
Phase 3 Combination
Phase 3 Combination
Advanced or Metastatic Solid Tumors with HER2 Expression (previously treated)
Phase 2 Monotherapy*
HER2+ Breast Cancer with HER2 Expression
Phase 1 Monotherapy and Combination
Phase 1b/2 Monotherapy
Phase 1b/2 Monotherapy
GI Cancers with HER2 Expression
Phase 1b/2 Monotherapy and Combination

 Back
Back



