Elranatamab
Overview + Rationale
- B-cell maturation antigen (BCMA) is a tumor necrosis factor receptor superfamily member that is expressed predominantly on mature B cells1
- BCMA mediates survival of antibody-secreting plasma cells after binding to its ligands BAFF (B-cell activating factor of the TNF family) and APRIL (a proliferation inducing ligand)2
- In multiple myeloma, BCMA is widely expressed on malignant plasma cells1
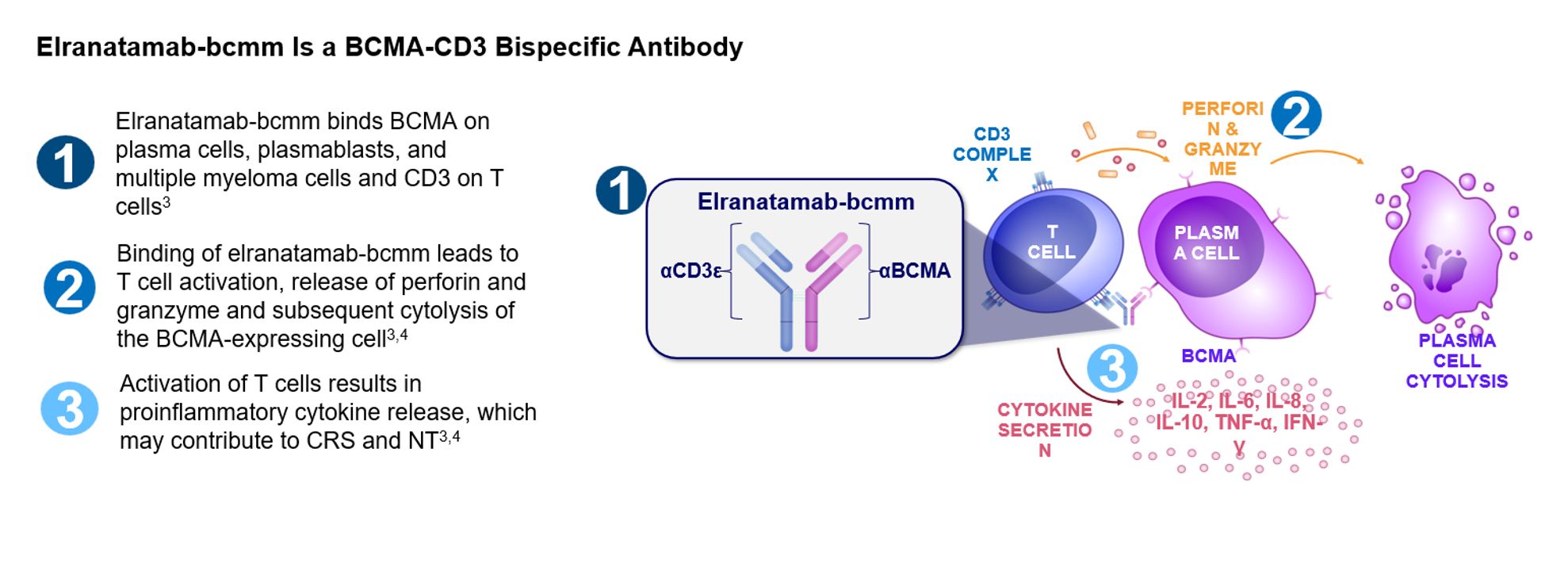
Mechanism of Action
Stage of Development
Elranatamab is being investigated as a single agent and in combination with other agent(s) in the following disease settings.
Relapsed/Refractory Multiple Myeloma
Phase 1B Combination
Phase 1B/2 Combination and Monotherapy*
Phase 2 Monotherapy*
Phase 3 Monotherapy
Phase 3 Monotherapy and Combination
Phase 1B/2 Combination and Monotherapy*
Phase 2 Monotherapy*
Phase 3 Monotherapy
Phase 3 Monotherapy and Combination
Newly Diagnosed Multiple Myeloma (Transplant Ineligible)
Phase 3 Combination
Newly Diagnosed Multiple Myeloma (Post-Transplant Maintenance)
Phase 3 Monotherapy
Multiple Myeloma Double–Class Exposed
Phase 3 Monotherapy and Combination

 Back
Back



