Enfortumab vedotin
Overview + Rationale
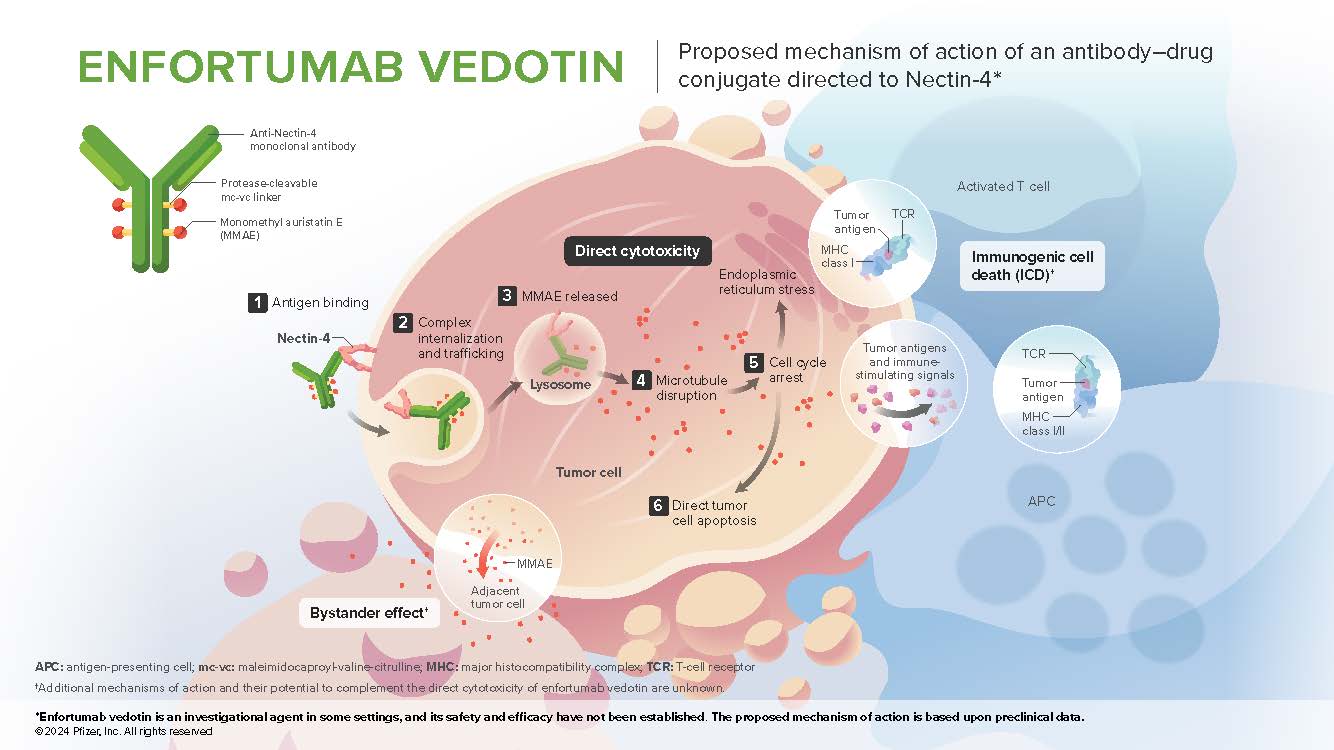
- Enfortumab vedotin (EV) is an antibody-drug conjugate directed to Nectin-4. It is made up of a fully human monoclonal antibody specific for Nectin-4, the microtubule disrupting agent monomethyl auristatin E (MMAE), and a protease-cleavable linker maleimidocaproyl-valine-citrulline that covalently attaches MMAE to the antibody which may enable preferential release of MMAE within target cells.1,2 Delivery of intracellular MMAE to tumor cells expressing Nectin-4 leads to cell cycle arrest and apoptotic cell death3
- Nectin-4 is a cell adhesion molecule that is expressed in both normal and tumor tissues and is involved in multiple cellular processes known to be associated with oncogenesis, including cell adhesion, migration, proliferation, differentiation, and survival4
- High expression of Nectin-4 in select solid malignancies, including pancreatic, lung, breast, ovarian, and esophageal cancers, is associated with poor prognosis5-11
- The elevated expression levels of Nectin-4 in urothelial carcinoma and other tumors, compared to its low-to-moderate expression in normal tissues, make it a robust therapeutic target for drug development4,5
Partner: Astellas Pharma Global Development, Inc.
Mechanism of Action
Stage of Development
Enfortumab vedotin is being investigated in the tumor types shown below. Safety and efficacy for the uses listed below have not been established.
Locally Advanced or Metastatic Urothelial Cancer
Phase 3 Combination†

 Back
Back




