PD1-IL12
PF-07921585 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
RATIONALE FOR CANCER TARGET:
- IL-12 is an established driver of T-cell differentiation and anti-tumor immunity, but previous attempts to use IL-12 as a target have demonstrated unacceptably high rates of toxicity1
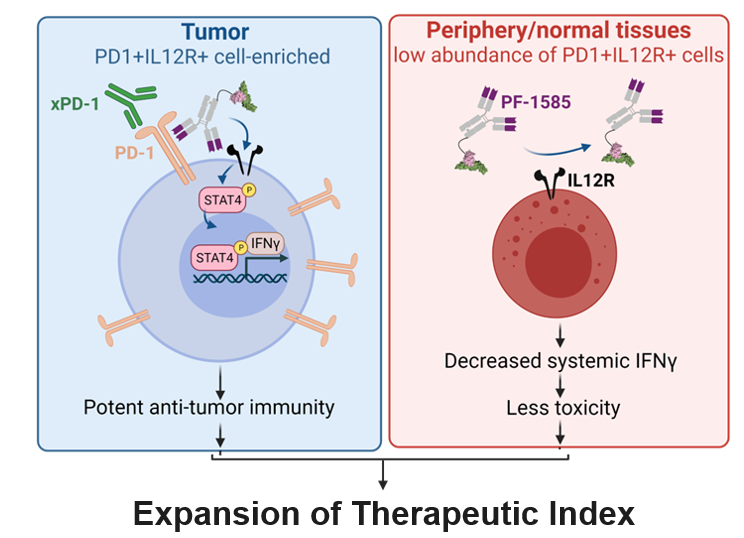
- IL-12 receptor agonism promotes anti-tumor immunity via direct enhancement of interferon-γ production and cytolytic capacity of CD8 T-cells and effects on CD4 T and NK cells2
- PD-1 is highly expressed on CD4+ and CD8+ TILs compared to circulating T-cell subsets2
OVERVIEW1
- PF-07921585 is a first-in-class fusion of a human IL-12 mutein and a bivalent antihuman PD-1 full-length, Fc-null IgG1 designed to target human PD-1-expressing immune cell subsets while permitting simultaneous binding of anti-PD-1 antagonist antibodies
- This antibody-cytokine fusion is designed to deliver IL-12 preferentially to PD-1 positive T cells in the tumor microenvironment, which may spare systemic effect, resulting in the potential for an improved therapeutic window
Mechanism of Action
In pre-clinical models, PF-07921585 targets a potency-reduced human IL-12 mutein to PD-1+ TILs with potential for anti-tumor immunity to drive anti-tumor immunity and could reduce IL-12 toxicities while also permitting simultaneous binding of PD-1 antagonists to enable combination with PDx therapy.
Stage of Development
Non-Small Cell Lung Cancer (NSCLC)
Phase 1 Monotherapy and Combination
Advanced and Metastatic Solid Tumors
Phase 1 Monotherapy and Combination

 Back
Back

