KAT6 Inhibitor
PF-07248144 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
- Functionally active fusions of Lysine Acetyltransferase 6A (KAT6A) with CBP1, p3002 or TIF23 have been described in AML and the resulting aberrant acetylation might contribute to the process of leukaemogenesis
- KAT6A contributes to the inhibition of cell senescence, and therefore to tumor cell immortality
- Inhibition of histone acetyltransferases KAT6A/B was shown to induce senescence and to arrest tumor growth in mouse embryonic fibroblasts
- PF-07248144 is a small molecule inhibitor of KAT6A
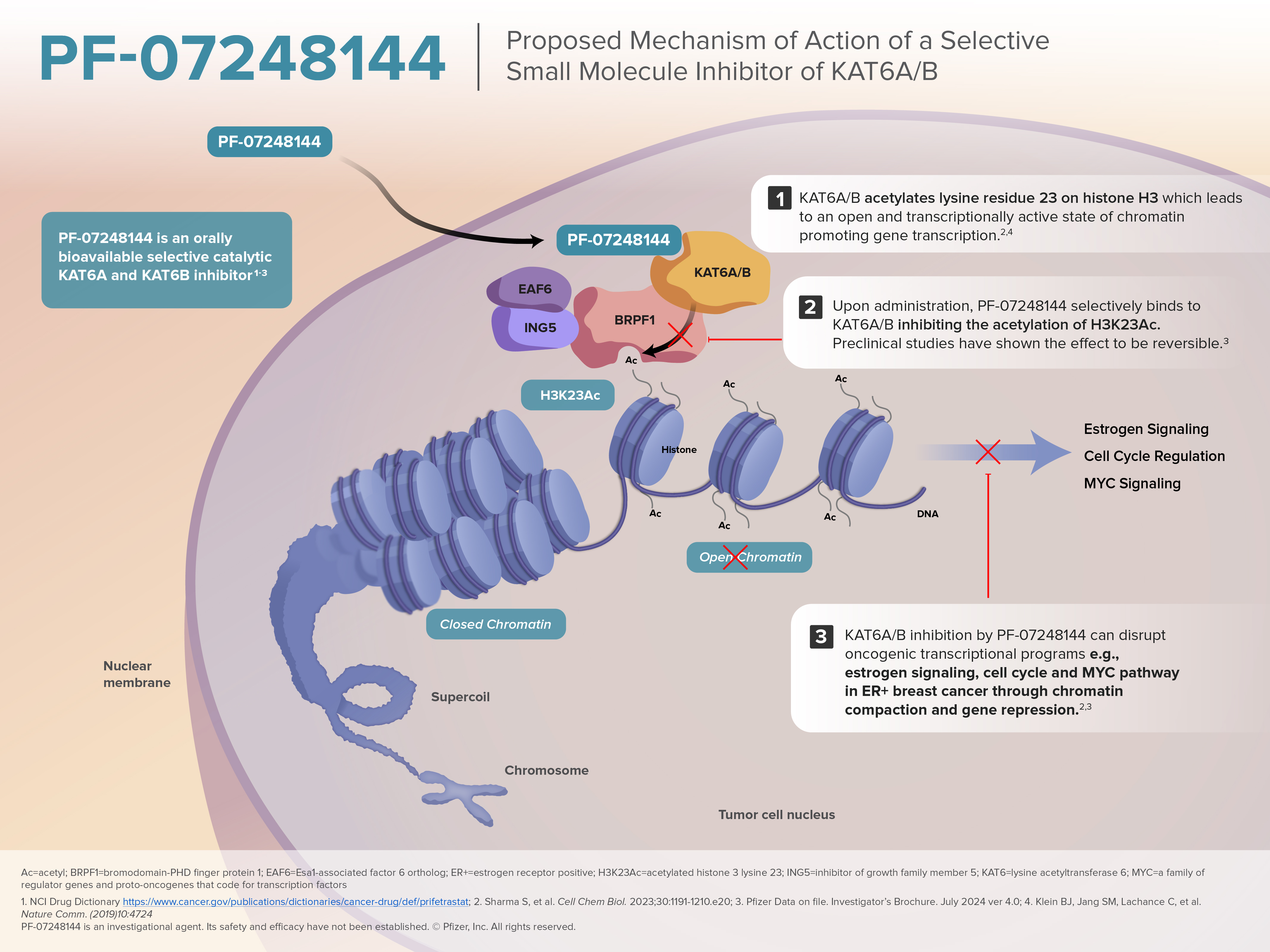
Mechanism of Action
- As part of the bromodomain- and PHD finger-containing protein 1 (BRPF1) complex, KAT6A acetylates lysine residue 23 on histone H3*
- H3K23 acetylation leads to a relaxed chromatin enabling gene transcription
- While acetylation by KAT6A potentially affects large parts of the DNA, certain transcription factors, as well as pre-existing epigenetic marks, were shown to recruit the BRPF1 complex for a more localized activity
Inhibition
Stage of Development
HR+/HER2- Metastatic Breast Cancer
Phase 3 Combination†
Phase 1 Monotherapy and Combination
Phase 1 Monotherapy and Combination

 Back
Back
