PD-L1-directed Antibody Drug Conjugate
PF-08046054 is an investigational compound. Its safety and efficacy have not been established
Overview + Rationale
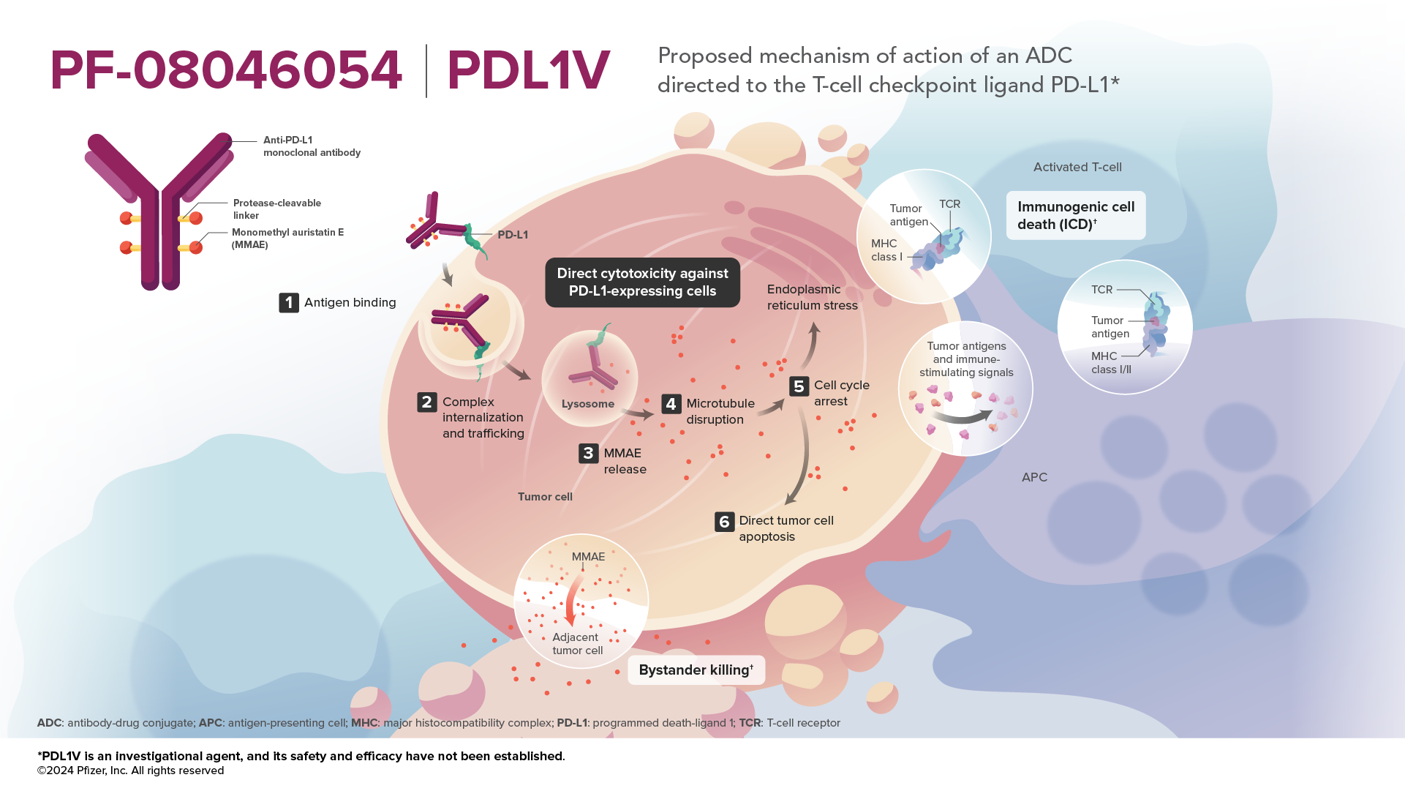
- PF-08046054 is an investigational antibody-drug conjugate (ADC) that contains 3 components: a monoclonal antibody directed to PD-L1 (programmed death ligand 1), a microtubule-disrupting agent MMAE (monomethyl auristatin E), and a protease-cleavable mc-vc (maleimidocaproyl-valine-citrulline) linker that covalently attaches MMAE to the antibody, which enables preferential release of MMAE within target cells1
- PD-L1 is a cell-surface protein primarily known for its role in the PD-1/PD-L1 immune checkpoint, which inhibits T-cell activation2-4
- Expression of PD-L1 in tumors can signal through PD-1 on T cells to inhibit T-cell effector function2
- PD-L1 expression is elevated in multiple solid tumors, including head and neck squamous cell carcinoma, non-small-cell lung cancer, melanoma, triple-negative breast cancer, urothelial cancer, cervical cancer, gastric cancer, ovarian cancer, and esophageal cancer1,2-17
- The elevated expression of PD-L1 in solid tumors relative to normal tissue makes it an ideal molecular target for ADCs1
- The proposed MOAs of PDL1V reflect the hallmarks of vedotin-based ADCs: MMAE-induced cytotoxicity against PD-L1-expressing tumor cells, immunogenic cell death (ICD), and bystander effect 1
- The PDL1V antibody has been designed to enable targeting of PD-L1 with an ADC through engineering for potential of increased internalization and payload delivery1
- While PDL1V targets the PD-L1 immune checkpoint ligand, nonclinical data suggests checkpoint inhibition through blockade of PD-1/PD-L1 interactions is unlikely to be a major contributor to the mechanism of action due to limitations of ADC-based dose level, schedule, and exposure1
Mechanism of Action
Stage of Development
Advanced Solid Tumors
Phase 1 Monotherapy and Combination
Thoracic Cancer & Squamous Cell Carcinoma of the Head and Neck (SCCHN)
Phase 1 Monotherapy and Combination

 Back
Back

