Binimetinib
Overview + Rationale
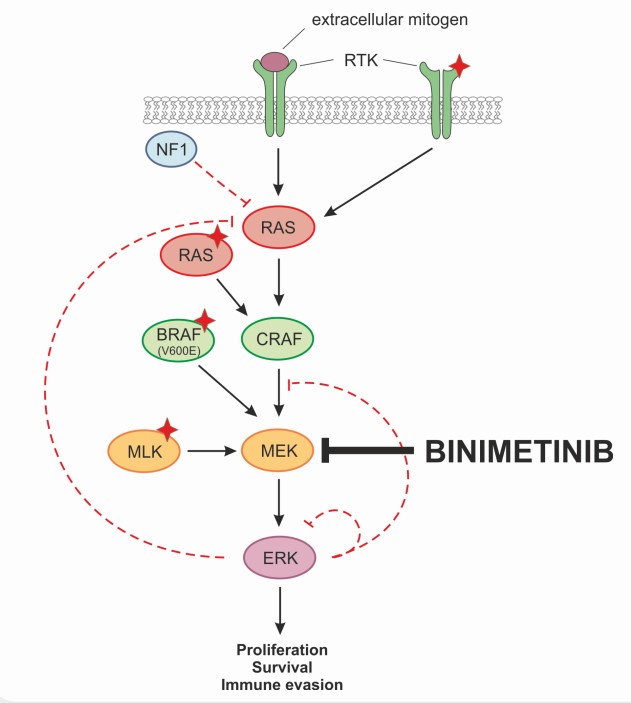
- The mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 are upstream regulators of the mitogen-activated protein kinase (MAPK) cascade
- This cascade regulates diverse cellular functions, including cell proliferation, survival, differentiation, and migration, and is deregulated in numerous cancer types
- BRAF inhibition in BRAF-mutant melanoma is limited by intrinsic and acquired resistance. Reactivation of the MAPK pathway drives BRAF-inhibitor resistance and is a contributor to treatment failure in BRAF-mutant melanoma
- Binimetinib is an orally available inhibitor of mitogen-activated protein kinase 1 and 2 (MEK1/2) with potential antineoplastic activity
- This may eventually lead to an inhibition of tumor cell proliferation and an inhibition in production of various inflammatory cytokines, including interleukin-1, -6, and tumor necrosis factor
Mechanism of Action
- Binimetinib is a reversible inhibitor of MEK1 and MEK2 activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway
- In vitro, binimetinib inhibited extracellular signal related kinase (ERK) phosphorylation in cell-free assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant (eg, V600e) human melanoma cell lines
- Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models
Stage of Development
Binimetinib is being investigated in combination with other agent(s) in the tumor types shown here. Safety and efficacy of binimetinib in combination for the uses listed below has not been established.
BRAF V600E/K-mutant+ Metastatic or Unresectable Locally Advanced Melanoma
Phase 3 (1L) Combination*

 Back
Back
