Brentuximab vedotin
Overview + Rationale
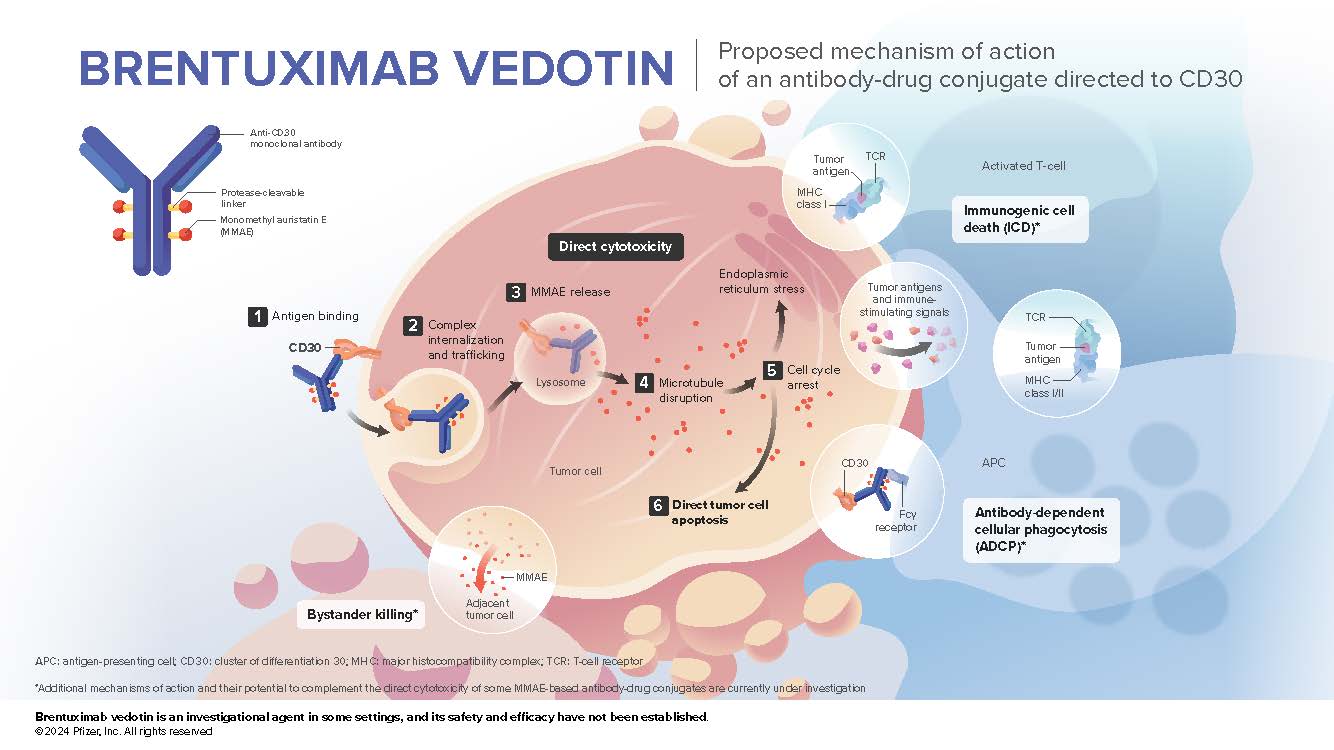
- Brentuximab vedotin is an antibody-drug conjugate composed of the antibody SGN-30, directed to human CD30,1,2 which is covalently attached to the microtubule-disrupting agent MMAE2,3 via a protease-cleavable mc-vc linker2-4
- CD30 is expressed in many B- and T-cell lymphomas while having limited expression in normal tissue1
Partner: Takeda Pharmaceutical Company Limited.
Mechanism of Action
Targeted delivery of MMAE to CD30- expressing tumor cells is the primary mechanism of action of brentuximab vedotin. 5 However, the direct cytotoxicity associated with brentuximab vedotin may be complemented by secondary effects, 6 including the bystander effect and several important immune-oncology related effects, such as immunogenic cell death 7-9 and antibody-dependent cellular phagocytosis. 10
Stage of Development
Brentuximab vedotin is being investigated in combination with other agent(s) in the tumor types shown here. Safety and efficacy for the uses listed below has not been established.





This information is current as of February 4th 2025.
*Closed to Enrollment.
REFERENCES: 1. Wahl AF. Cancer Res. 2002: 3736-42; 2. Francisco JA. Blood. 2003: 1458-65; 3. Doronina SO. Nat Biotechnol. 2003: 778-84; 4. Okeley NM. Clin Cancer Res. 2010: 888-97; 5. ADCETRIS prescribing information. June 2023; 6. Li F. Cancer Res. 2016: 2710-19; 7. Gardai SJ. Cancer Res. 2015: Abstract 2469; 8. Muller P. Cancer Immunol Res. 2014: 741-55; 9. Heiser R. Molec Cancer Ther. 2023: doi:10.1158/1535-7163.MCT-23-0118; 10. Oflazoglu E. Blood. 2007: 4370-2. 11. NCT02927769 https://clinicaltrials.gov/study/NCT02927769 Accessed January 16, 2025. 12. NCT04569032. https://clinicaltrials.gov/ct2/show/NCT04569032 Accessed January 16; 2025. 13. NCT04404283. https://clinicaltrials.gov/ct2/show/NCT04404283 Accessed January 16, 2026. 14. NCT04609566. https://clinicaltrials.gov/ct2/show/NCT04609566 Accessed January 16, 2025.

 Back
Back