STING Agonist
PF-07820435 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
- PF-07820435 is an orally delivered STING agonist being developed as a type 1 IFN inducer for a broad range of solid tumors3
- In vivo, antitumor activity was shown following PF-07820435 treatment of tumor-bearing hSTING-KI mice3
RATIONALE FOR CANCER TARGET:
- Insufficient cytotoxic T lymphocyte infiltration and the accumulation of immunosuppressive cells within tumors are factors in the inadequate clinical effectiveness of cancer treatment1
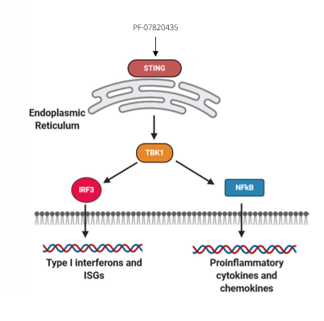
- The ‘stimulator of interferon genes’ (STING) is an endoplasmic protein that induces the production of pro-inflammatory cytokines such as type I interferons (IFNs)2
- The STING pathway drives activation of type I interferons and other inflammatory cytokines in the immune response against tumors2
- STING agonists lead to activation of downstream signaling via phosphorylation of TBK1 and IRF3 and induction of the type I IFN3
Mechanism of Action
PF-07820435-mediated STING induction is intended to induce both adaptive and innate immune responses resulting in improved tumor control by
- Inducing immune-stimulatory cytokines, enhancing antigen presentation, and T-cell activation;
- Increasing T-cell infiltration in TME; and
- Eliciting tumor cell death via T cell and NK cell mediated tumor cell killing
Data suggest that PF-07820435 can elicit type I IFN responses in PBMCs, myeloid cells and multiple tumor cell types in both healthy people and cancer patients.
Stage of Development
Advanced Solid Tumors
Phase 1 Monotherapy and Combination*

 Back
Back
