BRAFi
PF-07799933 is an investigational compound. Its safety and efficacy have not been established.
Overview + Rationale
RATIONALE FOR CANCER TARGET
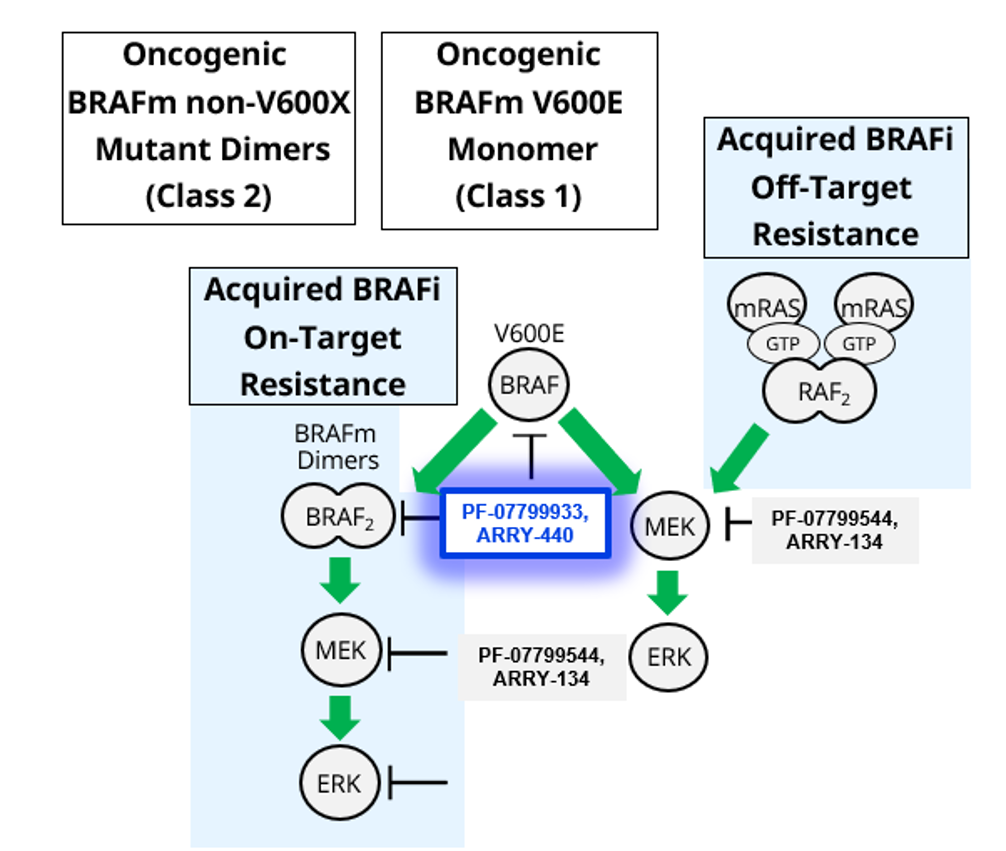
- BRAF, a serine/threonine protein kinase, plays a role in regulating the MAP kinase/ERKs signaling pathway, which may be constitutively activated due to BRAF gene mutations
- Oncogenic BRAFV600E signals as an active monomer in the absence of active RAS, however, in many tumors BRAF dimers mediate ERK signaling
- Class I BRAF inhibitors are thought to be inactive against BRAF altered dimers
- Approximately 15%-30% of resistance to Class I BRAF inhibitors in melanoma is driven by drug-acquired splice variants that signal as dimers
- Additionally, Class II and III BRAF alterations (alterations which signal as dimers) can occur de novo, as point mutations, fusions or deletions
- Patients with Class II/III (non-V600) mutations/alterations currently have no targeted therapeutic option and represent a population of unmet need
OVERVIEW
- PF-07799933 is a selective ATP-competitive small-molecule RAF kinase inhibitor, which has been shown to suppress the RAF/MEK/ERK pathway in tumor cells expressing BRAF V600-mutant (Class I) and non-V600-altered (Class II and III) kinase
- In various xenograft models, PF-07799933 has shown anti-tumor activity alone and in combination with clinically relevant targeted therapeutics and drug exposure in the brain
Mechanism of Action
- PF-07799933 binds to and inhibits BRAF Class I mutants (activated, monomeric V600 mutations), BRAF Class II mutants (activated, dimeric mutations), and BRAF Class III mutants (loss-of-function mutations that transactivate wildtype BRAF heterodimers)
- It is believed that PF-07799933 is also highly brain-penetrant
- PF-07799933 is highly selective for inhibition of only mutated BRAF, which is believed to provide a wide therapeutic safety index
Inhibition

 Back
Back
