Enzalutamide
Overview + Rationale
- Androgens are required for normal growth and function of the prostate
- Androgens promote the growth of both normal and cancerous prostate cells by binding to and activating the androgen receptor (AR), a protein that is expressed in prostate cells
- Once activated, the AR stimulates the expression of specific genes that cause prostate cells to grow
- Enzalutamide is an orally bioavailable, organic, nonsteroidals small molecule inhibitor of the AR with antineoplastic activity
- AR over-expression in prostate cancer represents a key mechanism associated with castration-resistant prostate cancer
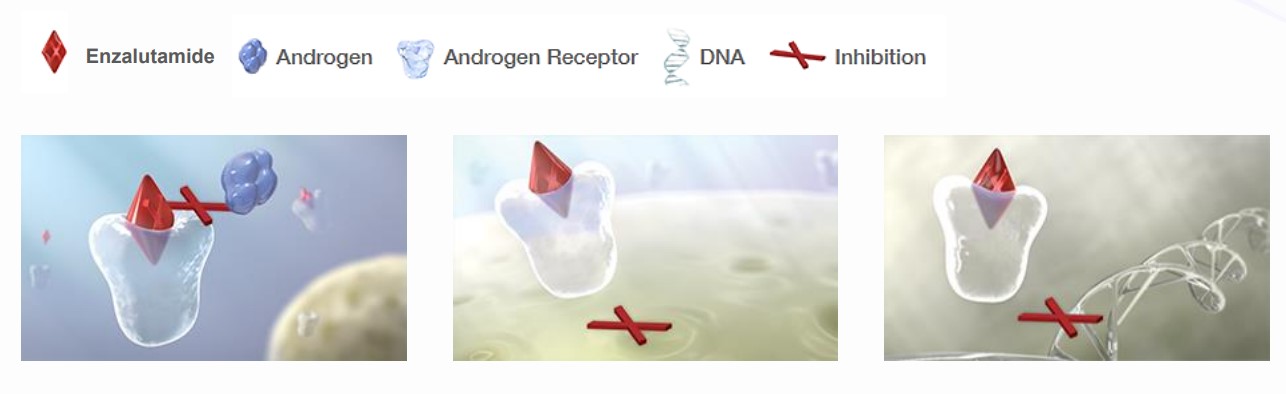
Mechanism of Action
- Enzalutamide is an AR inhibitor that acts on multiple steps of the AR-signaling pathway within the tumor cell
- In the cytoplasm, enzalutamide has been shown to inhibit androgen binding to the AR
- At the nuclear membrane, enzalutamide has been shown to inhibit the AR from entering the nucleus
- In the nucleus, enzalutamide has been shown to inhibit the AR binding to DNA
Androgen Receptor
Stage of Development
Enzalutamide is being investigated in combination in the tumor type shown here. Safety and efficacy of enzalutamide in combination with these compounds, for the use listed below, have not been established.
Prostate Cancer
Phase 1 Combination*
Phase 1 Combination**
Phase 3 Combination*
Phase 1 Combination**
Phase 3 Combination*
Metastatic Castration-Sensitive Prostate Cancer with DDR Gene Mutated
Phase 3 Combination§
Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Phase 3 Combination§

 Back
Back
